
One way is the transfer of electrons between two atoms until both atoms have octets. There are two ways for an atom that does not have an octet of valence electrons to obtain an octet in its outer shell. The tendency of an atom toward a configuration in which it possesses eight valence electrons is referred to as the “ Octet Rule.” In these lower energy states, the outermost energy level has eight electrons (an “octet”). This instability drives them toward the lower energy states represented by the noble gases that are nearby in the periodic table. The elements in the other groups have subshells that are not full, so they are unstable when compared to the noble gases.

They are already at a low energy state, so they tend to stay as they are.
POLYATOMIC ION BONDING FULL
These elements have electron configurations characterized by full s and p subshells. For atoms, these lower energy states are represented by the noble gas elements. Lower energy configurations are more stable, so things are naturally drawn toward them. Throughout nature, things that are high in energy tend to move toward lower energy states. Deviations from this ratio result in charged particles called ions. This one-to-one ratio of charges is not, however, the most common state for many elements. The overall charge on the atom is zero, because the magnitude of the negative charge is the same as the magnitude of the positive charge. This is because the number of electrons (negative in charge) is equal to the number of protons (positive in charge). Up until now we have been discussing only the elemental forms of atoms which are neutrally charged. 3.1 Introduction to the Octet Rule 3.2 Ions and the Periodic Table Common Cations Common Anions Ions of Transition Metals 3.3 Ionic Bonding 3.4 Practice Writing Correct Ionic Formulas 3.5 Naming Ions and Ionic Compounds 3.6 Polyatomic Ions 3.7 Naming Polyatomic Ions 3.8 Properties and Types of Ionic Compounds 3.9 Arrhenius Acids and Bases 3.10 Focus on the Environment – Acid Rain 3.11 Chapter Summary 3.12 References This text is published under creative commons licensing, for referencing and adaptation, please click here. The following video might help to memorize the polyatomic molecules.CH104: Chapter 3 – Ions and Ionic Compounds
*HCO 3 − Hydrogen carbonate (bicarbonate) More important polyatomic ions are marked with asterisk below. When a H + is added to a polyatomic ion, the negative charge of the ion is decreased by one unit.Įxample: PO 4 3- : phosphate ion, HPO 4 2-: hydrogen phosphate ion Example: NO 2 –: nitrite, NO 3 – : nitrate Lower number of oxygen containing polyatomic ion is named as -ite and higher number of oxygen containing polyatmic ions are named as -ate.

Oxo anions have end name either r -ate or -ite. That is why sometimes they are called oxo anion. Only two polyatomic anions that have end name -ide (OH –)are hydroxide and cyanide (CN –) Only one positively charged or polyatomic cation is NH 4 +. Many polyatomic anions have names that end in the suffix-ate or ite. The names of polyatomic cations end in the suffix -onium.

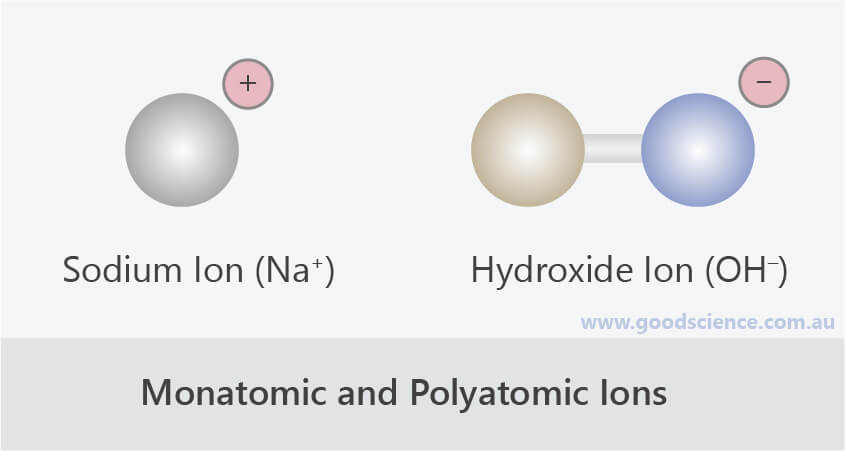
Some metalloids also form polyatomic ions. Central nonmetals are mainly from period 2 and 3 like N, S, P, Cl. In polyatomic ion, one central nonmetal attached to either oxygen or hydrogen. They are composed on more than one element. Polyatomic ions are charged species, consisting of group of atoms.


 0 kommentar(er)
0 kommentar(er)
